Latest research projects
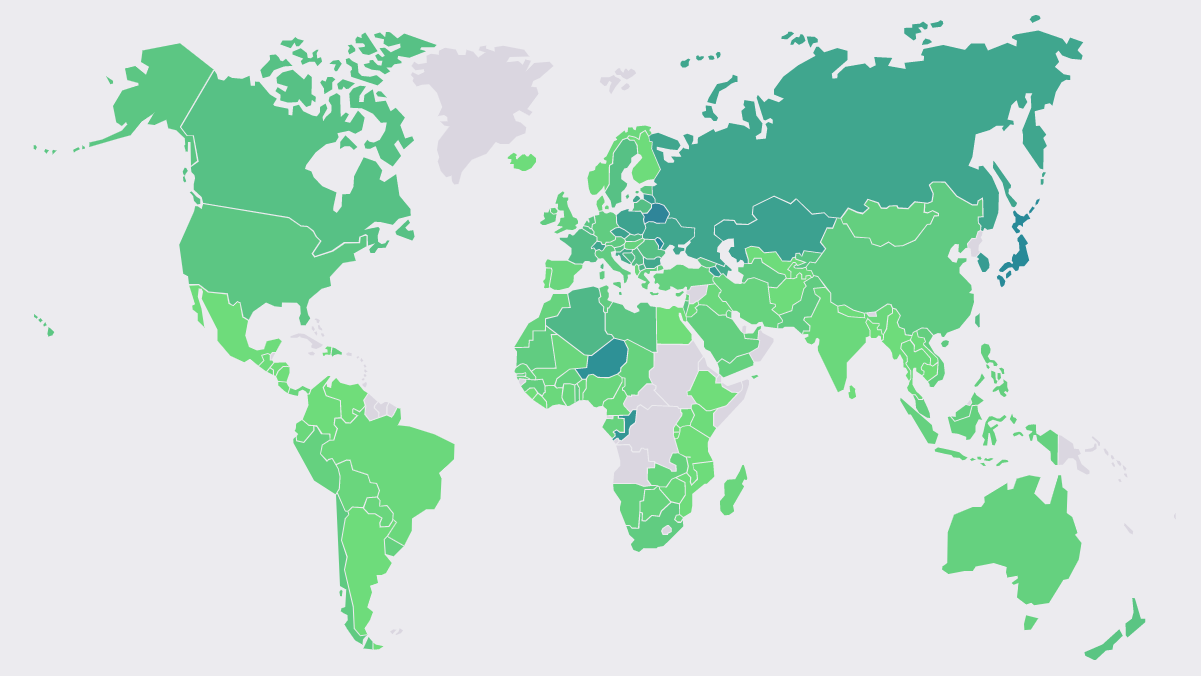
The VCP conducts global research, investigating the roots, trends over time and impacts of vaccine confidence at regional, national and sub-national levels.

We partnered with Wits Vida, the Vaccine Confidence Fund, and PHRU to study how much, if at all, social media and community influencers can influence and increase uptake of influenza vaccines.

Strengthen national training curricula and integrate targeted, culturally grounded interpersonal communication practices into training curricula for health workers in ECARO countries.

Mixed method study to examine adult and HCP confidence in vaccination in Eastern Europe (Balkans, Caucasus, Central Asia).

Mixed method study to explore risk perceptions and emotional determinants of health around COVID-19 and influenza vaccination in Europe.

VCP founder Heidi J. Larson named one of the most dynamic business women shaping the future for her role in building bridges of trust in the fight against misinformation and vaccine hesitancy.

In this Global Health Coffee Session at the Harvard Global Health Institute, VCP founder Heidi J. Larson unpacks how misinformation spreads and strategies for rebuilding public trust in public health in a time of doubt.
CIDRAP releases interim update on its Vaccine Integrity Project, spotlighting global threats to vaccine confidence and outlining next steps to support equitable immunization efforts worldwide.

A lot of people have genuine and legitimate questions and “you’re going to lose them if you immediately jump back and say ‘that’s silly’ or ‘that’s not the fact’”, says VCP founder Heidi J. Larson.